▪️ Demonstrating bioequivalence (BE) remains the key regulatory hurdle for generic drug approval. The high cost of running clinical BE trials is also a challenge.
▪️ As a result, some branded drugs remain on the market past the originator’s patent expiration, without cost-effective generic alternatives that could benefit patients.
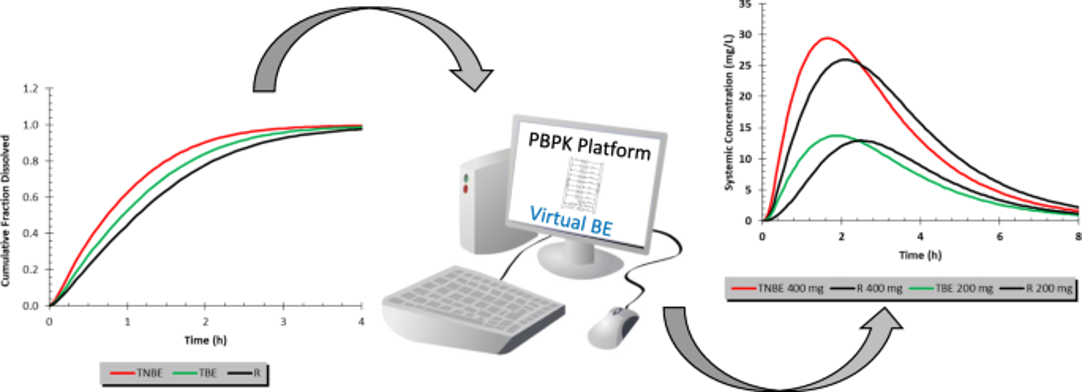
▪️ In silico modeling & simulation, specifically physiologically-based pharmacokinetics (PBPK) leveraging in vitro data, could be a cost-effective option in lieu of running an in vivo comparative clinical BE endpoint study.